Hennig Brand – 1669
The German alchemist Hennig Brand was the first to discover a new chemical element: phosphorus. He was attempting to create gold from urine when he obtained a substance that glowed in the dark. His discovery marked the beginning of the search for individual chemical elements.
Henry Cavendish – 1766
The English scientist discovered hydrogen, which he called “inflammable air,” through the reaction of a strong acid with metals such as zinc. It was later recognized as a fundamental element since it is part of water and other organic compounds.
Joseph Priestley – 1774
The British scientist Joseph Priestley isolated the gas we now know as oxygen, though he initially called it “dephlogisticated air.” He discovered it by heating mercuric oxide and observing that a gas was released which intensified a candle flame.
Antoine Lavoisier – 1789
He published a list of 33 known elements and classified them as metals and nonmetals. He also formulated the Law of Conservation of Mass, earning him the title of the father of modern chemistry.
Johann Wolfgang Döbereiner – 1829
Döbereiner expanded on his idea and officially published the Law of Triads, which would later help Mendeleev create the periodic table.
Döbereiner – 1817
He noticed that some elements had similar properties, grouping them into triads in which the middle element had a mass and properties between the other two—this was the first attempt to relate elements based on their characteristics.
Alexandre-Émile Béguyer de Chancourtois – 1862
Chancourtois created the first spiral periodic table, known as the “telluric helix,” organizing elements by atomic weight. This showed that elements with similar properties aligned vertically.
John Newlands – 1864
He proposed the Law of Octaves, stating that if elements are arranged by increasing atomic weight, every eighth element shares similar properties with the first.
Dmitri Mendeleev – 1869
A hundred years after the discovery of the first element, the chemist Mendeleev presented his periodic table of elements, organizing the 60 known elements by atomic mass and leaving empty spaces where he predicted the existence of yet undiscovered elements.
Lothar Meyer – 1871
He published a table almost identical to Mendeleev’s, but based on atomic volumes and physical properties, confirming the validity of the periodic system.
Paul Émile Lecoq de Boisbaudran – 1875
He discovered gallium, one of the elements Mendeleev had predicted, confirming the accuracy of Mendeleev’s predictions and giving prestige to his table.
Clemens Winkler – 1886
He discovered germanium, another element predicted by Mendeleev, reaffirming that the periodic system could anticipate new elements.
William Ramsay - 1894William Ramsay – 1894
He discovered argon and, along with other scientists, identified neon, krypton, xenon, and helium, forming the group of noble gases that were added as a new column in the table.
Wilhelm Röntgen – 1895
He discovered X-rays, and although not directly related to the periodic table, his work enabled advances in chemistry for studying atoms in greater detail.
Marie and Pierre Curie – 1900
Along with Henri Becquerel, they discovered radioactivity and radioactive elements such as polonium and radium, opening a new field of research on unstable elements.
Henry Moseley – 1913
He determined that elements should be arranged by atomic number rather than atomic mass, correcting previous errors.
Glenn T. Seaborg – 1940
He discovered ten transuranic elements, including lutetium, americium, and curium, and reorganized the table to include the actinide series.
Laboratories – 1990 to Present
International scientific teams discovered new, artificially created elements such as nihonium, moscovium, tennessine, and oganesson, completing the last spaces of the modern periodic table.
Origin of the Periodic Table
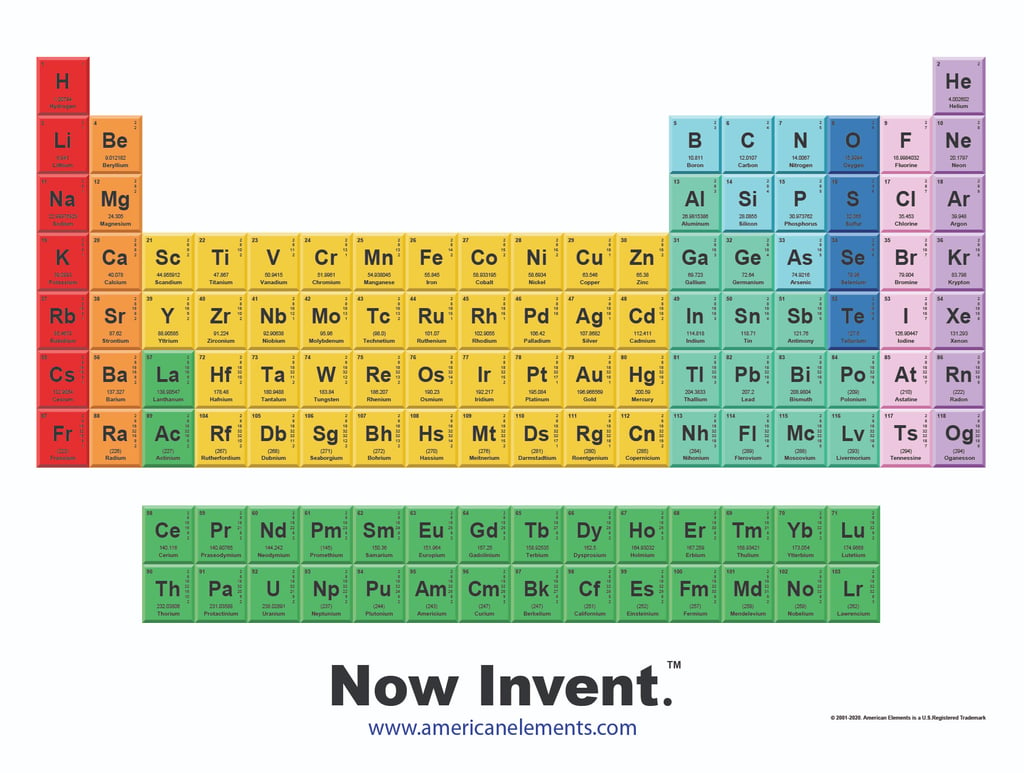
The origin of the periodic table dates back to the 19th century, when scientists began to notice patterns in the properties of known elements. The Russian chemist Dmitri Mendeleev was the first to organize the elements into a table in 1869, arranging them according to atomic mass and grouping them by similar chemical properties. What was most impressive was that he left empty spaces for undiscovered elements and accurately predicted their properties. Over time, the table was refined—especially when it was reorganized according to atomic number instead of mass—resulting in the modern format we use today.
Questions
Periodic Table of Elements
Damián Srulevich y Lucas Rodrigues